Calcium Chloride vs. Rock Salt. Which do you use?
Calcium Chloride vs. Rock Salt. Which do you use?
Before ordering the traditional rock salt for your ice preventive needs, take a look at all the pros of calcium chloride vs. rock salt.
Calcium chloride outdistances traditional deicing materials to achieve safer, bare pavement – faster than salt or abrasives alone. Calcium chloride melts up to eight times as much ice as does salt alone – within the first 30 minutes at 20F (-7C) following application. Pre-mixed with salt and abrasives, calcium chloride becomes a cost-effective edge for winter road safety.
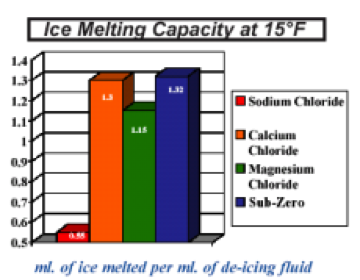
Highway Safety: studies show that, in 85% of applications, calcium chloride/ salt mixtures achieve bare pavement faster than salt alone at temperatures near 30F (-1C), to ease traffic and reduce accidents.
- Savings: calcium chloride increases salt’s effectiveness, therefore reducing the number of applications necessary during storms – saving manpower, equipment and material costs. Plus, it freeze-proofs abrasives to help them embed in ice and snow, so you lose less material to spreader bounce and traffic scattering,
- Exothermic: Calcium chloride releases heat to activate salt’s melting ability.
- Hygroscopic: Calcium chloride attracts moisture required for rock salt’s melting action.
- Fast acting: Calcium chloride begins to dissolve immediately upon application to break the bond between pavement and ice.
- Low eutectic point: calcium chloride melts at much lower temperatures than salt.
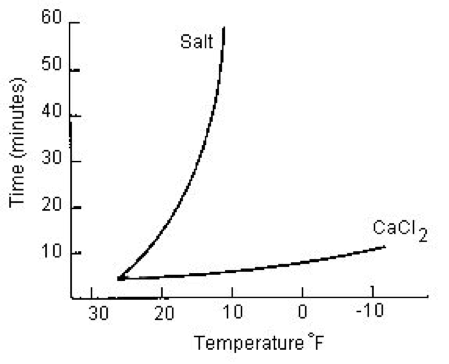
The Pros of using Calcium Chloride
Calcium chloride is effective down to a temperature of -25 degrees Fahrenheit and is some two to 13 times faster than other products. Although it is more expensive than some other deicers and similar in price to magnesium chloride, pound for pound it melts twice as much ice, according to Peters Chemical Co. Also, its effect on vegetation, corrosive characteristics, and concrete performance compares favorably to competing ice-melt materials, including magnesium and potassium chloride. In industrial and highway applications it is often applied as a liquid. Together with cheaper solutions, such as rock salt, it significantly improves performance. (Rock salt by itself is effective to about 15 degrees Fahrenheit.)
There is some agreement among researchers that the major damage to the pavements is due to frequent freeze/thaw cycles. Deicers with lower freeze points reduce the number and frequency of freeze/thaw cycles the pavement would go through during the winter months. Both calcium chloride and magnesium chloride have lower freeze points. They are less damaging to the pavements when compared with sodium chloride because calcium chloride has a lower freezing point.
Summary
Calcium chloride is an effective deicer, working at temperatures below most competing products, and is significantly more effective than sodium chloride because of its ability to extract moisture from its surroundings and to cause exothermic or heat generating reactions. Also when mixed with traditional rock salt, Calcium Chloride greatly improves the effectiveness of the deicing material.
Dan Murphy
